Overview
Overview
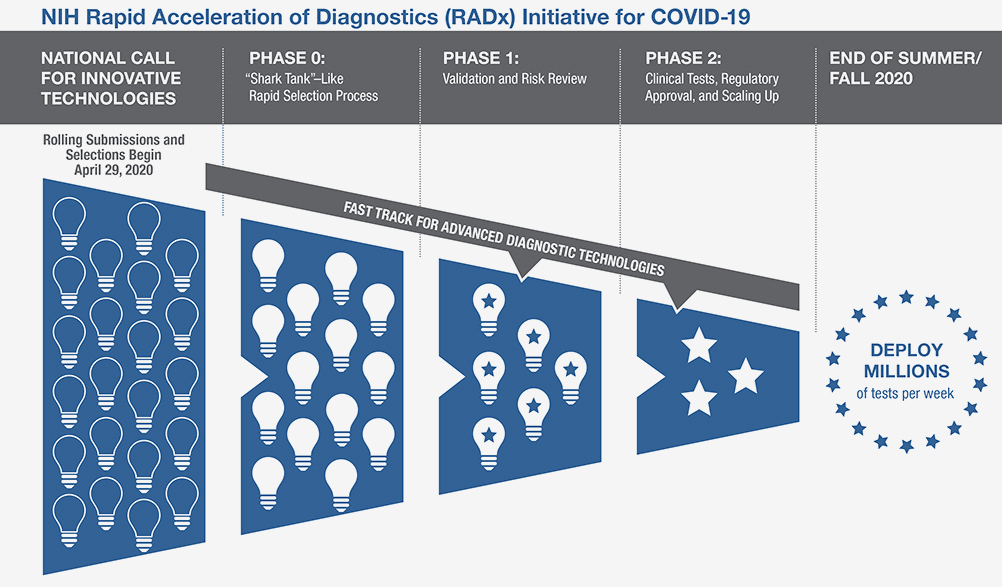
The Rapid Acceleration of Diagnostics (RADx) initiative invests in innovative technologies as a way to speed development of rapid and widely accessible COVID-19 testing. Finalist technologies will be matched with technical, business, and manufacturing experts to increase odds of success.
Usability evaluations of the novel technologies will be conducted by CACP’s HomeLab, in partnership with The Atlanta Center for Microsystems Engineered Point-of-Care Technologies (ACME-POCT). These evaluations can begin at the design phase of the process and will conclude with user testing for the final product. User testing will occur wherever the novel test is intended to be used: in the home, in a clinic, in a pharmacy, or in any appropriate community setting. Evaluations will focus on the individual who administers a COVID-19 test.
For more information contact Sarah Farmer
Participant FAQ
Participant FAQ
What is the project about?
The Rapid Acceleration of Diagnostics (RADx) initiative invests in innovative technologies as a way to speed development of rapid and widely accessible COVID-19 testing. RADx Tech initiative https://www.nih.gov/research-training/medical-research-initiatives/radx/radx-programs#radx-tech “aims to speed the development, validation, and commercialization of innovative point-of-care and home-based tests, as well as improve clinical laboratory tests, that can directly detect the virus. RADx Tech will expand the Point-of-Care Technologies Research Network (POCTRN) established several years ago by NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB). The network will use a flexible, rapid process to infuse funding and enhance technology designs at key stages of development, with expertise from technology innovators, clinical testing, regulatory affairs, entrepreneurs, and business leaders.”
Who is conducting the work?
Homelab https://cacp.gatech.edu/research/accessibility/HomeLab a unit of the Center for Advanced Communications Policy (CACP), at Georgia Tech, is a residence-based network of older adults who have agreed to participate in research studies in their home. Generally, participants are age 55-99; with a variety of medical conditions are represented, from health to those who have conditions such as COPD, diabetes, congestive heart failure, arthritis, and other normative age-related declines. In addition, HomeLab Kids is made up of child participants, with a focus on childhood medical conditions (asthma, juvenile diabetes, special dietary needs, ADHD, Autism spectrum disorder, and chronic diseases).
What is HomeLab’s approach?
Usability evaluations of the novel technologies are conducted by CACP’s HomeLab,https://cacp.gatech.edu/research/accessibility/HomeLab in partnership with The Atlanta Center for Microsystems Engineered Point-of-Care Technologies (ACME-POCT). These evaluations can begin at the design phase of the process and will conclude with user testing for the final product. User testing will occur wherever the novel test is intended to be used: in the home, in a clinic, in a pharmacy, or in any appropriate community setting. Evaluations will focus on the individual who administers a COVID-19 test.
How does HomeLab conduct Usability Evaluations?
As is the standard procedure, HomeLab obtain Georgia Tech Institutional Review Board (IRB) approval for the conduct of all research involving human subjects. RADx usability research protocols and recruitment processes are tailored based on the specifics of each novel test and the tasks involved for each test. This ensures that all participants are protected and can be confident that the protocols follow best practice safety and ethical guidelines.
For each test, 15-20 participants will be recruited, and a projected 10-20 tests will pass through the program. Overall, between 150 and 400 participants will participate in the program. Participants will be recruited to be representative of the intended user population for each test.
Usability evaluations will be conducted for all of the pertinent contexts: in a home setting; primary care clinics, hospital emergency rooms, and pharmacies, in a clinical lab, or in the community, depending on the intended use location of the tests.
I’m interested in knowing more – who do I contact?
https://cacp.gatech.edu/research/accessibility/HomeLab.
https://cacp.gatech.edu/research/accessibility/HomeLab
For more information on participation:
Georgia Tech HomeLab
500 10th Street NW
Atlanta, GA 30332 - 0620
Phone: 404-385-4614
Industry FAQ
Industry FAQ
What is the project about?
The Rapid Acceleration of Diagnostics (RADx) initiative invests in innovative technologies as a way to speed development of rapid and widely accessible COVID-19 testing. RADx Tech initiativehttps://www.nih.gov/research-training/medical-research-initiatives/radx/radx-programs#radx-tech “aims to speed the development, validation, and commercialization of innovative point-of-care and home-based tests, as well as improve clinical laboratory tests, that can directly detect the virus. RADx Tech will expand the Point-of-Care Technologies Research Network(POCTRN) established several years ago by NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB). The network will use a flexible, rapid process to infuse funding and enhance technology designs at key stages of development, with expertise from technology innovators, clinical testing, regulatory affairs, entrepreneurs, and business leaders.”
How does RADx work?
The NIH invites scientists and inventors with a candidate rapid testing technology to compete in a national “shark tank”-type COVID-19 testing challenge. The program has up to $500 million allocated to fund the various phases of development. The technologies will undergo a highly competitive, rapid three-phase selection process to identify the best candidates for at-home or point-of-care tests for COVID-19. Finalists will be matched with technical, business, and manufacturing experts to increase the odds of success. If certain selected technologies are already relatively far along in development, they can be advanced immediately to the appropriate step in the commercialization process. Details and application information are available on the POCTRN site: https://www.poctrn.org/radx
Who is conducting the work?
HomeLab https://cacp.gatech.edu/research/accessibility/HomeLab a unit of the Center for Advanced Communications Policy (CACP), at Georgia Tech, is a residence-based network of older adults who have agreed to participate in research studies in their home. Generally, participants are age 55-99; with a variety of medical conditions are represented, from health to those who have conditions such as COPD, diabetes, congestive heart failure, arthritis, and other normative age-related declines. In addition, HomeLab Kids is made up of child participants, with a focus on childhood medical conditions (asthma, juvenile diabetes, special dietary needs, ADHD, Autism spectrum disorder, and chronic diseases).
What is HomeLab’s approach?
Usability evaluations of the novel technologies are conducted by CACP’s HomeLab, https://cacp.gatech.edu/research/accessibility/HomeLab in partnership with The Atlanta Center for Microsystems Engineered Point-of-Care Technologies (ACME-POCT). These evaluations can begin at the design phase of the process and will conclude with user testing for the final product. User testing will occur wherever the novel test is intended to be used: in the home, in a clinic, in a pharmacy, or in any appropriate community setting. Evaluations will focus on the individual who administers a COVID-19 test.
What is HomeLab’s overall evaluation process?
HomeLab will conduct a three-pronged evaluation:
- Human Factors Analysis
- Expert Heuristic Evaluation
- Usability Evaluation
If the test under evaluation is deemed to have significant usability flaws at the end of the Human Factors Analysis, project directors will be briefed and given the opportunity to terminate the evaluation for that test
What is HomeLab’s Human Factors Evaluation process?
HomeLab conducts a Design Failure Modes and Effects Analysis (DFMEA) in which the tasks required to complete the testing process will be identified, as well as potential errors for each task. Following this, the frequency of potential errors will be estimated as well as the associated impact of each potential error. During usability testing, the frequency of each error will be validated and the DFMEA will be updated. HomeLab will also estimate cognitive, sensory, and physical workloads associated with the use of each kit.
What is HomeLab’s Expert Heuristic Evaluation?
Two or more HomeLab researchers independently review each test kit in a lab setting to identify features of the test likely to cause errors, as well as features of the test that may cause other usability issues.
HomeLab researchers utilize a heuristic evaluation tailored to each test based on FDA usability guidance, sound human factors design principles, and lab experience in the design and evaluation of medical devices.
How does HomeLab conduct Usability Evaluations?
As is the standard procedure, HomeLab obtain Georgia Tech Institutional Review Board (IRB) approval for the conduct of all research involving human subjects. RADx usability research protocols and recruitment processes are tailored based on the specifics of each novel test and the tasks involved for each test.
For each test, 15-20 participants will be recruited, and a projected 10-20 tests will pass through the program. Overall, between 150 and 400 participants will participate in the program. Participants will be recruited to be representative of the intended user population for each test.
Usability evaluations will be conducted for all of the pertinent contexts: in a home setting; primary care clinics, hospital emergency rooms, and pharmacies, in a clinical lab, or ; in the community, depending on the intended use location of the tests.
What are the Metrics used in the Usability Evaluations?
HomeLab will report, at a minimum, on the following metrics:
- Usability: The extent to which a test can be used by specified users to achieve specified goals with effectiveness, efficiency, and satisfaction in a specified context of use.
- Effectiveness: Is the accuracy and completeness with which users achieve specified goals. This includes: percent task completion, frequency and type of errors, frequency of required assistance from evaluator to complete task or test, frequency of user access to documentation or guidance.
- Efficiency: The resources expended in relation to the accuracy and completeness with which users achieve goals; the time it takes to complete a task, and the mean time taken to complete a test
- Satisfaction: Elements of satisfaction include: lack of discomfort, and positive attitudes toward the use of a test
- Context of use: This includes an assessment of the users, tasks, equipment, and physical environment in which evaluation takes place; the setting and type of space used for usability evaluation, and, relevant circumstances that could affect results.
I’m interested in knowing more – who do I contact?
For information on HomeLab, see https://cacp.gatech.edu/research/accessibility/HomeLab.
For a description of capabilities see https://cacp.gatech.edu/research/accessibility/HomeLab
For more information:
Georgia Tech HomeLab
500 10th Street NW
Atlanta, GA 30332 - 0620
Phone: 404-385-4614

